Faculty Profile
Theodore Dibble


Professor
Department of Chemistry
421 Jahn Lab
Education
Ph.D., 1992, University of Michigan.
Postdoctoral, Wayne State (1992-1994), Purdue (1994-1995), and Caltech (1995-1996).
Research Overview
The Dibble Group uses computational chemistry to investigate the mechanisms and kinetics of reactions important for energy and the environment. The current focus is redox chemistry of mercury in the atmosphere. Other major interests include aerosols (nucleation, surface reactions, and bulk chemistry), dissolved organic matter in aquatic/marine systems, and organic chemistry in the atmosphere. We work with experimentalists to test our results and we work with atmospheric modelers to determine how our predictions influence our understanding of global and regional processes.
Atmospheric Mercury Chemistry
Mercury is a neurotoxin. It is transferred from the atmosphere to ecosystems upon oxidation from Hg(0) to Hg(II). Oxidation is initiated by Br or OH radicals:
HO + Hg → HOHg
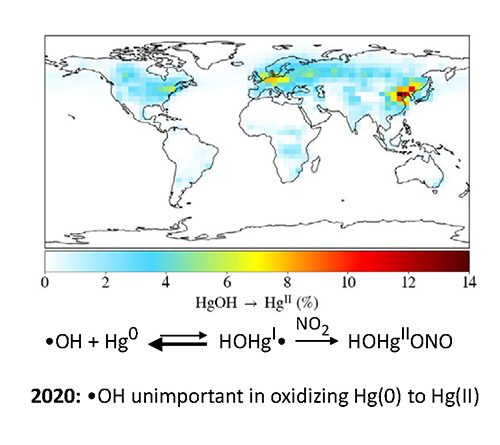
Some models still assume that the OH + Hg reaction produces stable Hg(II) compounds. In 2020 we showed that HOHg fell apart so fast (see the Figure, below) that it only contributed 1% to global Hg oxidation. But we later discovered that Hg(I) radicals (both HOHg and BrHg) reacted with ozone, e.g.:
HOHg + O3 → HOHgO• + O2
This may enable OH to initiate about 1/3 of total oxidation of atomic mercury to Hg(II). HOHgO is pretty reactive, and we are still investigating its influence of atmospheric mecury cycling. .
Atmospheric Organic Chemistry
We have investigated the chemistry of alkyl, alkoxy, and peroxy radicals (R•, RO•, and ROO•, respectively). We maintain an interest in isoprene chemistry and the unknown mechanism by which ROO• + NO makes RONO2. Atmospheric organic chemistry also brings up fundamental issues in chemistry, such how intra- and inter-molecular hydrogen bonds involving radical centers affect reactivity and enable new chemistry. With computational chemistry we can investigate a wide range of these problems!
More Information
Follow me on Google Scholar, ResearchGate, or Twitter, or email me if you want further information about my research!
Follow this link for Information on Graduate Admissions in Chemistry. Follow this link to learn more our M.S. and Ph.D. programs in Environmental Chemistry.
Selected Publications
Complete Listing (Google Scholar)
-
Nitrate Radical Cannot Initiate Oxidation of Hg (0) to Hg (II) in the Laboratory or at Ground Level in the Atmosphere. D. H. Tharika Edirappulige, L. Cheng, P. J. Castro, and T. S. Dibble, manuscript under review.
- Atmospheric Chemistry of HOHg(II)O• Mimics That of a Hydroxyl Radical. D. T. Hewa Edirappulige, I. J. Kirby, C. K. Beckett, and T. S. Dibble, J. Phys. Chem. A 2023, 127, 8392-8403.
- Together, Not Separately, OH and O3 Oxidize Hg(0) to Hg(II) in the Atmosphere. P. J. Castro, V. Kello, I. Cernušák,and T. S. Dibble, J. Phys. Chem. A 2022, 126, 8266–8279.
- Combined Experimental and Computational Kinetics Studies for the Atmospherically Important BrHg Radical Reacting with NO and O2. R. Wu, P. J. Castro, C. Gaito, K. Beiter, T. S. Dibble, and C. Wang, J. Phys. Chem. A 2022, 126, 3914–3925.
- Improved mechanistic model of the atmospheric redox chemistry of mercury, V. Shah et al., Env. Sci. Technol. 2021, 55, 14445-14456.
- Modeling the OH-Initiated Oxidation of Mercury in the Global Atmosphere Without Violating Physical Laws, T. S. Dibble, H. L. Tetu, and Y. Jiao, C. P. Thackray, and D. J. Jacob, J. Phys. Chem. A 2020, 124, 444-453.
Current Graduate Advisees
 Weiyao Gu
Weiyao Gu
wgu100@syr.edu
- Degree Sought: PHD
- Graduate Advisor(s): Dibble
- Area of Study: FCH Environmental Chemistry
 Darshi Hewa Edirappulige
Darshi Hewa Edirappulige
dthewaed@syr.edu
- Degree Sought: PHD
- Graduate Advisor(s): Dibble
- Area of Study: FCH Environmental Chemistry