THE AMERICAN CHESTNUT PROJECT
Moving Closer to 10,000 Trees
Activities undertaken between November 1, 2019 and November, 30, 2020
This has been a challenging year for everyone due to the COVID-19 pandemic, including our American chestnut Research and Restoration Program at SUNY ESF. But our team has risen to the occasion and safely made significant progress, and is pleased to share these highlights with you.
Outcrossing pollinations and tissue culture - Hannah Pilkey (Production Manager)

Figure 1: View from rented lift over pollinated mother trees. Our team has no fear of heights.
This pollination season was an exceptional year for incorporating new mother trees and pollen types to promote genetic diversity in the transgenic breeding plan. At ESF's permitted sites, 1,400 nuts were produced through controlled pollinations (fig.1). In total, 41 mother trees were pollinated with pollen produced from 14 different transgenic trees under high-light conditions. These nuts will include pure American chestnut T2s and T3s. Note the common numbering format used for transgenics is T0 for the first transformed generation, then T1, T2, T3, etc. for the crosses. In addition to the American crosses, a few European, Chinese, and chinquapin transgenic hybrids were also produced for testing. Twenty-eight mother trees were pollinated with 5 different T2 transgenic pollen types to produce over 600 nuts that make up the T3 generation of outcrosses. In addition to ESF's pollinations, outcrosses were also performed by our TACF collaborators in Indiana, Maine, Vermont, and Virginia. A grand total of 4,845 nuts comprising the T3 generation of outcrosses were produced this summer. Approximately half of these nuts will inherit OxO and comprise the T3 generation, which will be the most genetically diverse, blight-tolerant offspring thus far. OxO expression assays have begun and will continue through the new year to identify which nuts inherited the OxO gene.
Despite challenges with COVID restrictions, clonal propagation and production of transgenic trees continued in the tissue culture lab. Over 100 transgenic T2 trees were multiplied, rooted, and made it to planting in the field this year. These trees have also begun producing pollen in the high light growth chamber, which will be freezer stored and used in the 2021 pollination season. Additionally, four new transgenic trees, two of which are from Virginia and Indiana, were also initiated into tissue this year. These trees have now entered the rooting phase of production and will contribute to diversifying plantings and pollen production in the upcoming year.
Development of new transgenic lines to aid diversity breeding -Linda McGuigan (Tissue culture lab manager)
In the fall of 2019, an experiment was conducted to transform American chestnut somatic embryos with the p35S-OxO gene. The experiment included five chestnut backgrounds (Alessi T2-1A, AxW3-46B, Bass Mountain #5-1B, Moss Lake 90016 C-21-1C, and TG-8A) and three methods of selection (semisolid medium in Petri plates, liquid medium in RITA temporary immersion bioreactors, and liquid medium in We Vitro containers). No significant differences were found between the treatments, however, 123 new transgenic lines were produced. These lines are currently being regenerated into shoot cultures. Once these lines have been tested for insert copy number, keeping only lines with single gene copies, then testing for level of OxO gene expression, keeping only the lines that express high enough to confer blight tolerance, they will be used to supplement the diversity outcrossing. Since these lines will have the gene inserted in a different location of the chestnut's genome, this eliminates possible problems from "linkage drag" and their diverse genomes will contribute to diversification and local adaptation of the restoration population. The following paper was published to help others use these techniques in the future:
McGuigan, L., Fernandes, P., Oakes, A., Stewart, K., & Powell, W. (2020). Transformation of American Chestnut (Castanea dentata (Marsh.) Borkh) Using RITA Temporary Immersion Bioreactors and We Vitro Containers. Forests, 11(11), 1196.

Figure 2. American chestnut somatic embryos during the transformation process. (A) Somatic embryos on desiccation plates. (B) Selection of embryo clumps on semisolid medium in Petri plates. (C-E) Selection of embryo clumps in We Vitro containers with liquid medium. (D) Aluminum foil covers the We Vitro containers. (E) A tea bag is used to keep the embryo clumps from moving into the liquid medium in the We Vitro containers when they are angled up. (F, G) Selection of embryo clumps in a RITA bioreactor with liquid medium.
Mother Tree Seed Production Orchards and Holding Plots - Jeff Zarnowski & Sean Satchwell (Field Manager and Assistant Field Manager)
Five areas expanded for chestnut plantings for production of trees for the public and for long-term demonstration/research plantings. To protect all the new plantings, an additional 1.7 miles of deer fence was installed. There are currently over 1,600 "Darling" American chestnut among over 3,600 transgenic and non-transgenic chestnut trees in our fields. These numbers will continue to increase each year through outcrossing and the development of new lines. We hope that after the COVID-19 restrictions are lifted we can begin public tours of this exciting field expansion. Please add a visit to your "to do" list for next summer.
- Plot A1. 4.47 acres of shelterwood plantings. Experiments on the best treatments for restoration to forested areas.
- Plot A2. 3.8 acres of shelterwood plantings.
- Plot B. 4.2 acres Demo Orchards containing the "Darling" American chestnut - Four orchard types: open field hardwood forest restoration with mixed chestnut/oak/hickory forest trees, chestnut/grape co-culturing, pure transgenic American chestnut orchards, and hybrid transgenic orchards.
- Plot C. 1.7 acres expanded for production containing "mother' trees, one- to three-year-old "Darling" American chestnut tree holding plots, and a common garden experiment comparing the "Darling" trees to other chestnut trees.
- Leonard Property Effective Pollination Distance study - 0.42 acres.
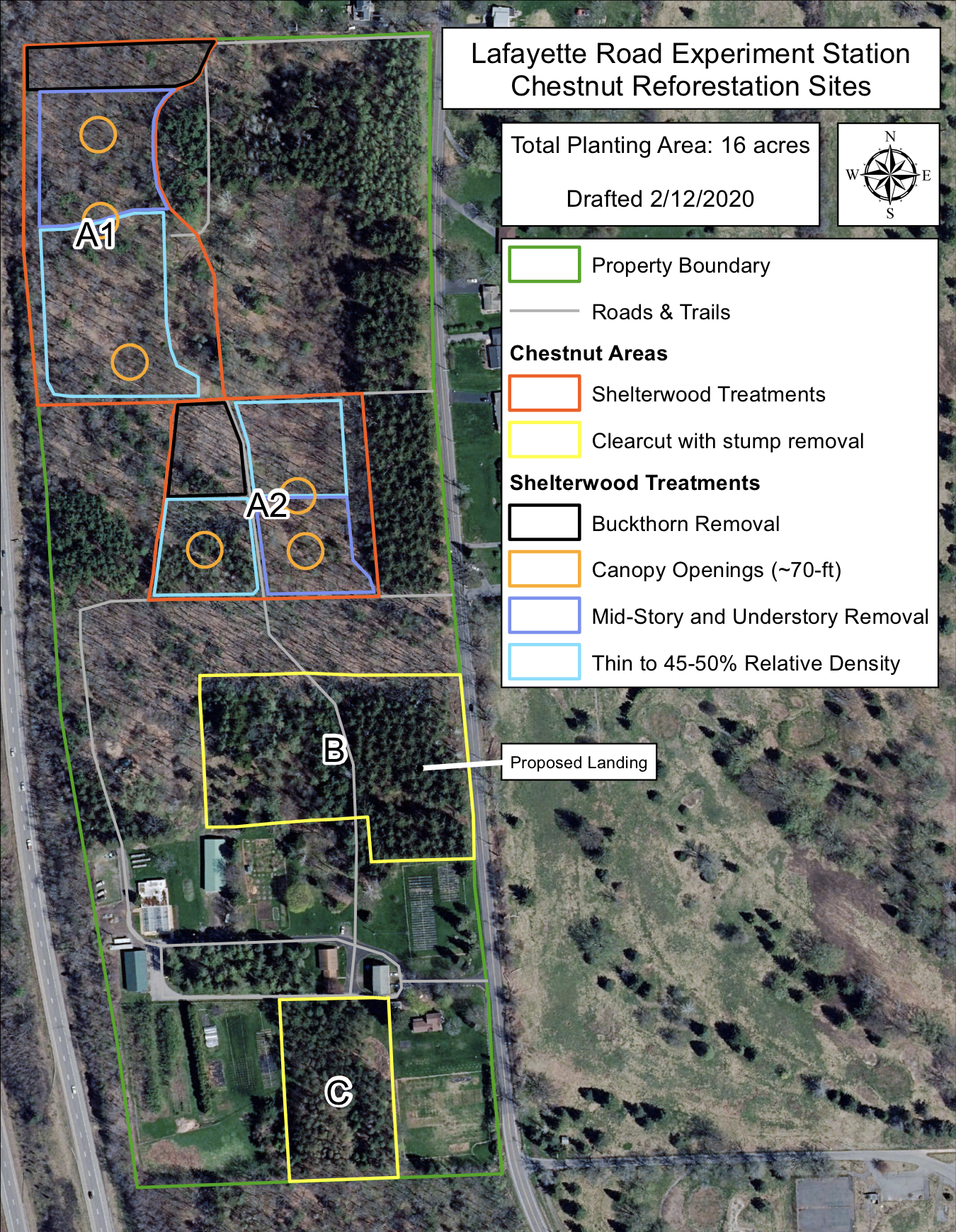
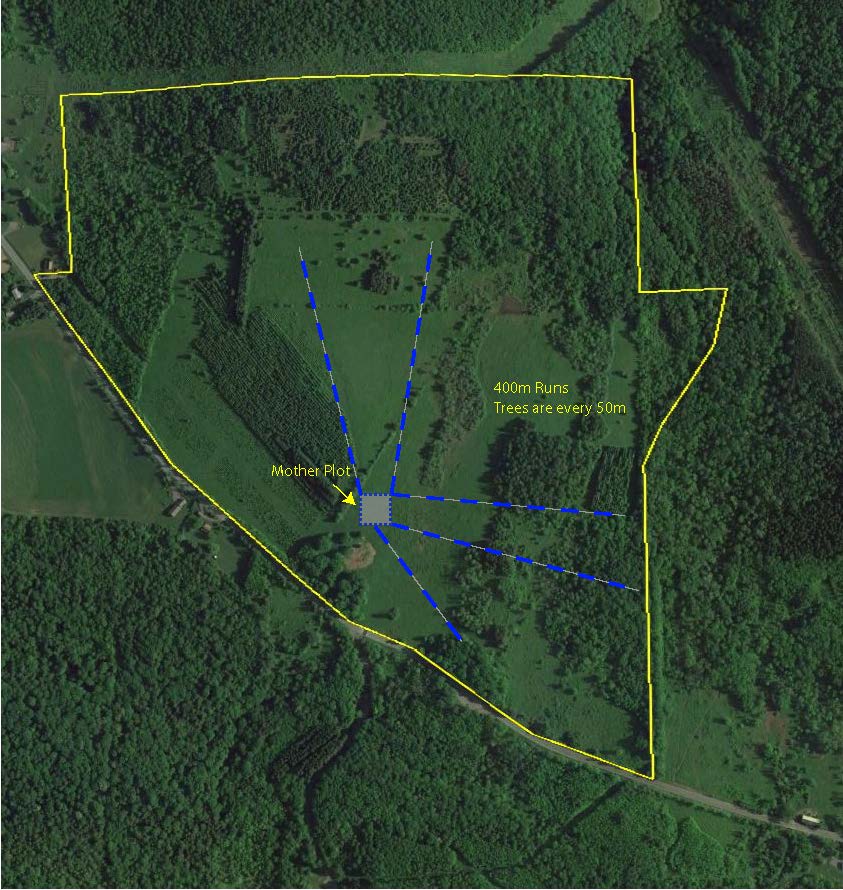
Figure 3. Left is a satellite map of SUNY ESF's Lafayette Road Experiment Station with the new planting areas marked and labeled. Right is the Leonard Property at Heiberg Memorial Forest showing the runs of chestnut sentinel trees radiating out from a small, "Darling" American chestnut orchard that will allow us to test the effective pollination distance for restoration plantings

Figure 4. To care for the significantly expanding chestnut plantings and prepare for public distribution of trees, a new John Deere w/loader with tree spade to move larger trees was purchased.
Final words from Bill Powell (Professor and Chestnut Project Director)
We have a great team working to get you the best, blight tolerant American chestnut trees possible. Although the progress may at times seem slow, especially with the regulatory parts of the project, we are actually making good progress considering we are doing something new and innovative. Being first means we have to blaze the path for others who wish to use the powerful tools of biotechnology to save other threatened trees and improve our forest's health. I am very proud of our team of researchers and students who keep their focus on the goal, even with the many challenges brought by the pandemic. One, of many, good examples is the virtual pollination workshop held this past summer to help the general public learn how to outcross the "Darling" American chestnut to wild-type mother trees in their area.
I am also proud of all our supporters that contributed financially as well as offering their time when we really needed it. The recent USDA open comment period was a great example. We had 2,689 unique, positive comments submitted about our petition for nonregulated status (the complete petition can be read here). I sincerely thank everyone who submitted a comment and I hope you will do it again during at least two more opportunities that we will be posting. The regulators have never seen this much support from the public. Keep up the good work and we will be able to restore the American chestnut.
