The American Chestnut Project
Environmental Interactions with Transgenic American Chestnuts
Environmental Interactions with Transgenic American Chestnuts Andrew E. Newhouse and William A. Powell With gracious acknowledgement to many research collaborators SUNY College of Environmental Science & Forestry, Syracuse, NY
Background
Darling transgenic American chestnuts have shown significantly enhanced tolerance to the chestnut blight fungus, and will soon be evaluated by federal regulatory agencies before potential public distribution. The oxalate oxidase enzyme expressed in Darling transgenic American chestnuts is naturally found in many food crops and wild plants, and it protects the tree by degrading a toxin rather than by killing the fungus, so it is unlikely to present novel risks when expressed in American chestnut. However, along with data from molecular, growth, and nutritional tests, it is valuable for regulators and prudent for environmental scientists to carefully evaluate potential environmental risks before deploying new restoration material. Here we summarize several comparative risk assessments of environmental interactions with transgenic American chestnuts and non-transgenic controls.
Leaf Herbivory by Insects
 Aquatic mayfly larvae performed better on American chestnut leaves than oaks and other
trees in a preliminary test1, so chestnut re-introduction may benefit stream ecosystems. Subsequent studies have
shown that caddisfly larvae survival was not significantly different on transgenic
vs. wild-type American chestnut leaves2. Separate studies of terrestrial (gypsy moth) caterpillars showed leaf consumption
differences between Chinese and American leaves, but similar consumption between wild-type
American and transgenic or B3F3 leaves.
Aquatic mayfly larvae performed better on American chestnut leaves than oaks and other
trees in a preliminary test1, so chestnut re-introduction may benefit stream ecosystems. Subsequent studies have
shown that caddisfly larvae survival was not significantly different on transgenic
vs. wild-type American chestnut leaves2. Separate studies of terrestrial (gypsy moth) caterpillars showed leaf consumption
differences between Chinese and American leaves, but similar consumption between wild-type
American and transgenic or B3F3 leaves.
Bumble Bees and Pollen
 Chestnuts are often considered to be wind-pollinated, but recent research has shown
that insects (including native bees) contribute to successful pollination13-15. Bumble bees were reared in microcolonies (photo at right) and supplied with chestnut
pollen containing the oxalate oxidase enzyme. There were no differences in survival,
body size, pollen use, or reproduction when bees were exposed to a field-realistic
concentration of oxalate oxidase in pollen16.
Chestnuts are often considered to be wind-pollinated, but recent research has shown
that insects (including native bees) contribute to successful pollination13-15. Bumble bees were reared in microcolonies (photo at right) and supplied with chestnut
pollen containing the oxalate oxidase enzyme. There were no differences in survival,
body size, pollen use, or reproduction when bees were exposed to a field-realistic
concentration of oxalate oxidase in pollen16.
Germination of Nearby Native Seeds
 Seeds of several native plant types from American chestnut habitats (Elymus/grass,
Cichorium/forb, Gaultheria/shrub, Acer/deciduous tree, Pinus/conifer) were germinated
in potting mix with different types of chestnut leaf litter3. Pinus seeds showed reduced germination in the presence of one wild-type control
leaf type compared to no-leaf trays, and Cichorium showed reduced biomass in B3F3
leaves compared to Darling leaves, but there were no significant differences in seedling
germination rates or total biomass when grown in transgenic vs wild-type American
chestnut leaf litter.
Seeds of several native plant types from American chestnut habitats (Elymus/grass,
Cichorium/forb, Gaultheria/shrub, Acer/deciduous tree, Pinus/conifer) were germinated
in potting mix with different types of chestnut leaf litter3. Pinus seeds showed reduced germination in the presence of one wild-type control
leaf type compared to no-leaf trays, and Cichorium showed reduced biomass in B3F3
leaves compared to Darling leaves, but there were no significant differences in seedling
germination rates or total biomass when grown in transgenic vs wild-type American
chestnut leaf litter.
Leaf Litter Decomposition
 Leaf decomposition rates were compared by placing different chestnut leaf types (wild-
type and transgenic American, and BC1 hybrid) in mesh bags on the forest floor. All
leaf types lost ~90% of their mass after 1 year; rates were not significantly different4.
Leaf decomposition rates were compared by placing different chestnut leaf types (wild-
type and transgenic American, and BC1 hybrid) in mesh bags on the forest floor. All
leaf types lost ~90% of their mass after 1 year; rates were not significantly different4.
Transgenic chestnut leaves were also tested for persistence of oxalate oxidase enzyme activity after leaf drop in the fall. Enzyme activity essentially ceased as soon as leaves turned brown (approximately one week after leaf drop in outdoor conditions), though activity could be preserved for more than a month in artificial freezer conditions5.
Transgene Inheritance
 If or when transgenic American chestnuts are deployed for restoration purposes, their
ecological significance ultimately will rely on inheritance of the transgene by offspring.
Meaningful genetic diversity in a potential restoration population will be accomplished
primarily by outcrossing transgenic trees with surviving wild chestnuts17. Since the
transgene is only present on one half of the chromosome, about half the offspring
from a cross with one transgenic parent are expected to be transgenic. This has been
confirmed in analyses of two generations of Darling offspring: 56 of 115 nuts tested
to date show transgene activity. Growth rates and survival of these transgenic offspring
are equivalent to their non-transgenic siblings (that didn't inherit the transgene),
and Cryphonectria parasitica inoculations indicate that transgenic offspring have
blight tolerance similar to their transgenic parent.
If or when transgenic American chestnuts are deployed for restoration purposes, their
ecological significance ultimately will rely on inheritance of the transgene by offspring.
Meaningful genetic diversity in a potential restoration population will be accomplished
primarily by outcrossing transgenic trees with surviving wild chestnuts17. Since the
transgene is only present on one half of the chromosome, about half the offspring
from a cross with one transgenic parent are expected to be transgenic. This has been
confirmed in analyses of two generations of Darling offspring: 56 of 115 nuts tested
to date show transgene activity. Growth rates and survival of these transgenic offspring
are equivalent to their non-transgenic siblings (that didn't inherit the transgene),
and Cryphonectria parasitica inoculations indicate that transgenic offspring have
blight tolerance similar to their transgenic parent.
Tadpoles in Vernal Pools
 Wood frog tadpoles were raised in individual jars with different types of deciduous
leaf litter to simulate an interaction that could take place in vernal pools18. Chestnut leaves (transgenic American, wild-type American, hybrid, or Chinese) were
not detrimental to tadpole survival compared to sugar maple controls; only American
beech leaves increased tadpole mortality. Development and growth rates were similar
on most leaf types, but American chestnut leaves (both transgenic and wild type) allowed
slightly increased growth rates compared to other leaf types when supplemental food
was not present, suggesting American chestnut restoration could conceivably benefit
amphibians.
Wood frog tadpoles were raised in individual jars with different types of deciduous
leaf litter to simulate an interaction that could take place in vernal pools18. Chestnut leaves (transgenic American, wild-type American, hybrid, or Chinese) were
not detrimental to tadpole survival compared to sugar maple controls; only American
beech leaves increased tadpole mortality. Development and growth rates were similar
on most leaf types, but American chestnut leaves (both transgenic and wild type) allowed
slightly increased growth rates compared to other leaf types when supplemental food
was not present, suggesting American chestnut restoration could conceivably benefit
amphibians.
Mycorrhizal Fungi
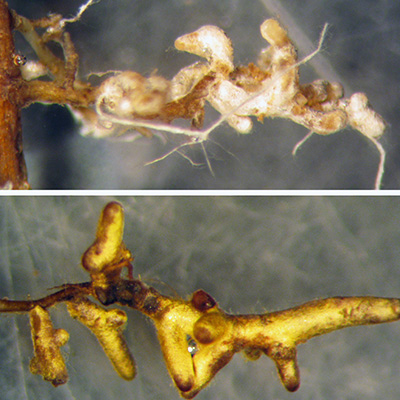 Several experiments have examin ed mycorrhizal association s with transgenic Ame rican
chestnut roots. Most recently, Darling roots were observed to be colonized at the
same rate (>95% of root tips colon ized) as wild- type American chestnut controls3 . These results corrob orate older studies on transgenic chestnut (inc luding both
greenhouse6 and field7 experiments) and studies on other transgenic trees (aspen , elm , Eucalyptus10, poplar11, and apple12), all of which indicate transgene presence does not inhibit mycorrhizal associations.
Several experiments have examin ed mycorrhizal association s with transgenic Ame rican
chestnut roots. Most recently, Darling roots were observed to be colonized at the
same rate (>95% of root tips colon ized) as wild- type American chestnut controls3 . These results corrob orate older studies on transgenic chestnut (inc luding both
greenhouse6 and field7 experiments) and studies on other transgenic trees (aspen , elm , Eucalyptus10, poplar11, and apple12), all of which indicate transgene presence does not inhibit mycorrhizal associations.
Conclusions
Some environmental interaction experiments show differences between Chinese and American chestnuts, which is not surprising given the differences between these species. However, neither B3F3 nor transgenic OxO-expressing American chestnuts have shown significant ecological differences compared to wild-type American chestnuts, apart from blight tolerance. Thus Darling transgenic American chestnuts do not appear to present greater ecological risks than traditional breeding.
References
1.Sweeney, B. and Jackson, D. (2016) Personal communication to W. Powell
2.Brown, A.J. (2017). M.S. Thesis. SUNY-ESF, Dept. of EFB.
3.Newhouse, A.E., Oakes, A.D., et al. (2018). Frontiers in Plant Science 9, 1–9.
4.Gray, A. (2015). M.S. Thesis. SUNY-ESF, Dept. of FNRM.
5.Matthews, D., Baier, K., and Powell, W. 2013 unpublished.
6.D'Amico, K.M., Horton, T.R., et al. (2015). Appl. Env. Microbiol. 81, 100–108.
7.Tourtellot, S.G. (2013). M.S. Thesis. SUNY-ESF, Dept. of EFB.
8.Kaldorf, M., Fladung, M., et al. (2002). Planta 214, 653–660.
9.Newhouse, A.E., Schrodt, F., et al. (2007). Plant Cell Reports 26, 977–987.
10.Lelmen, K.E., Yu, X., et al. (2010). Plant Biotechnology 27, 339–344.
11.Danielsen, L., Lohaus, G., et al. (2013). PLOS ONE 8, e59207.
12.Schäfer, T., Hanke, M.-V., et al. (2012). Genet. Mol. Biol. 35, 466–473.
13.de Oliviera, D., Gomes, A., et al. (2001) ActaHort. 561, 269-273.
14.Hasegawa, Y., Suyama, Y., et al. (2015). PLOS ONE 10 (3): e0120393.
15.Zirkle, C. (2017) M.S. Thesis. University of Arkansas.
16.Newhouse, A.E., Allwine, A., et al. (2019 in prep)
17.Steiner, K.C., Westbrook, J.W., et al. (2017). New Forests 48, 317–336.
18.Goldspiel, H., Newhouse, A.E., et al. (2018). Restoration Ecologydoi:10.1111/rec.12879.

