The American Chestnut Project
American chestnut tissue culture and transformation
American chestnut tissue culture
Plant tissue culture (also known as micropropagation) is a method of propagating plants asexually in aseptic conditions. The four main steps of micropropagation are establishment, multiplication, rooting and acclimatization. An additional step, regeneration, is needed for embryo tissue culture. The following explains the process of tissue culture for American chestnut somatic embryos (non-zygotic embryos established from zygotic cells). All steps are performed in a laminar-flow hood that uses a HEPA filter to keep the air aseptic. The whole process can take between 12 to 18 months.
Establishment
 To establish American chestnut somatic embryos in tissue culture, the nut is removed
from the spiny bur and then sterilized with bleach to rid it of microorganisms. The
cleaned nut is cut open and the ovules are removed and placed on an embryo initiation
medium (a semi-solid gel containing all of the essential mineral elements that the
plant would normally get from the soil, vitamins, sugar and hormones) in a sterilize
Petri dish. After a few weeks, many of the embryos will either become contaminated,
die or form callus that cannot be regenerated. Some of the embryos, however, will
begin to grow. The somatic embryos that survive are now ready to be multiplied.
To establish American chestnut somatic embryos in tissue culture, the nut is removed
from the spiny bur and then sterilized with bleach to rid it of microorganisms. The
cleaned nut is cut open and the ovules are removed and placed on an embryo initiation
medium (a semi-solid gel containing all of the essential mineral elements that the
plant would normally get from the soil, vitamins, sugar and hormones) in a sterilize
Petri dish. After a few weeks, many of the embryos will either become contaminated,
die or form callus that cannot be regenerated. Some of the embryos, however, will
begin to grow. The somatic embryos that survive are now ready to be multiplied.
Multiplication
 Once an embryo is established, it can be multiplied. By using a certain combination
of hormones in the medium, new embryos will begin to grow from the original one. As
more embryos develop, the nutrients in the medium become exhausted. The new embryos
are separated into smaller groups and transferred to fresh medium. Every two to three
weeks, as more embryos grow, they are separated and transferred to fresh medium.
Once an embryo is established, it can be multiplied. By using a certain combination
of hormones in the medium, new embryos will begin to grow from the original one. As
more embryos develop, the nutrients in the medium become exhausted. The new embryos
are separated into smaller groups and transferred to fresh medium. Every two to three
weeks, as more embryos grow, they are separated and transferred to fresh medium.
Regeneration
 The next step is to regenerate the embryos into shoots. To do this, the concentration
of nutrients, sugar and hormones in the medium is changed. There are a total of three
different media used during this process. Once the shoots develop, they are multiplied
on yet another medium. The shoots are cut into smaller segments and transferred to
fresh medium once a month.
The next step is to regenerate the embryos into shoots. To do this, the concentration
of nutrients, sugar and hormones in the medium is changed. There are a total of three
different media used during this process. Once the shoots develop, they are multiplied
on yet another medium. The shoots are cut into smaller segments and transferred to
fresh medium once a month.
Rooting
 When the shoots are 2 to 3 inches tall, they are ready to be rooted. They are dipped
into a liquid solution of a rooting hormone and then placed in the dark for four days.
Anywhere from 10 to 60 percent of the shoots going through this process will develop
roots. These rooted plants are put in potting mix and then they go through an acclimatization
process.
When the shoots are 2 to 3 inches tall, they are ready to be rooted. They are dipped
into a liquid solution of a rooting hormone and then placed in the dark for four days.
Anywhere from 10 to 60 percent of the shoots going through this process will develop
roots. These rooted plants are put in potting mix and then they go through an acclimatization
process.
Acclimatization
 This has proven to be the most difficult part of American chestnut tissue culture.
The plants are very sensitive to slight environmental changes and will die under the
wrong circumstances. The plantlets are placed in a controlled environment growth chamber
with bags kept over them to reproduce the high humidity they were used to in tissue
culture. The bags are removed in stages to let the plants get acclimated to lower
humidity slowly. They are watered and fertilized when needed. Forty to sixty percent
of plantlets going through acclimatization survive to be planted in the field.
This has proven to be the most difficult part of American chestnut tissue culture.
The plants are very sensitive to slight environmental changes and will die under the
wrong circumstances. The plantlets are placed in a controlled environment growth chamber
with bags kept over them to reproduce the high humidity they were used to in tissue
culture. The bags are removed in stages to let the plants get acclimated to lower
humidity slowly. They are watered and fertilized when needed. Forty to sixty percent
of plantlets going through acclimatization survive to be planted in the field.
American Chestnut Transformation
 To add potential blight-resistance genes to the American chestnut genome, a method
known as Agrobacterium-mediated transformation is being used. This method uses a bacterium called Agrobacterium tumefaciens, which is a natural genetic engineer. Wild-type Agrobacterium lives in the soil and colonizes small wounds near the root collar of many plant species.
When the Agrobacterium encounters a wound, it attaches itself to plant cells, pokes a microscopic hole into
the cell and injects small pieces of DNA. The DNA travels to the nucleus and is incorporated
into the chromosomes of the plant. Wild-type Agrobacterium injects genes that cause the plant's cells to divide rapidly, producing a warty gall,
and to produce food that only the Agrobacterium can use. Scientists have "tamed" Agrobacterium strains so that they insert genes of interest to the research project instead of the
genes that are only advantageous to the bacterium.
To add potential blight-resistance genes to the American chestnut genome, a method
known as Agrobacterium-mediated transformation is being used. This method uses a bacterium called Agrobacterium tumefaciens, which is a natural genetic engineer. Wild-type Agrobacterium lives in the soil and colonizes small wounds near the root collar of many plant species.
When the Agrobacterium encounters a wound, it attaches itself to plant cells, pokes a microscopic hole into
the cell and injects small pieces of DNA. The DNA travels to the nucleus and is incorporated
into the chromosomes of the plant. Wild-type Agrobacterium injects genes that cause the plant's cells to divide rapidly, producing a warty gall,
and to produce food that only the Agrobacterium can use. Scientists have "tamed" Agrobacterium strains so that they insert genes of interest to the research project instead of the
genes that are only advantageous to the bacterium.
To transform American chestnut somatic embryos, we use this Agrobacterium-mediated transformation method. This means we use a "disarmed" strain of Agrobacterium containing our putative resistance enhancing gene and a selectable marker gene. Initially, we used a scorable marker gene that codes for Green Fluorescent Protein (GFP) to help develop our protocol. We are now able to transform chestnut without the GFP gene. To perform the transformation, the Agrobacterium is mixed together with the chestnut embryos in a test tube. The embryos are placed on a desiccation plate (a sterile Petri plate with a slightly moistened filter paper) and the bacterium is given enough time to inject the designer DNA into the plant cells. Then the embryos are moved to a medium containing antibiotics that will kill the bacterium but not the plant cells. After a week, the embryos are transferred to a periodic immersion bioreactor with a liquid medium containing antibiotics. The antibiotics will eliminate plant cells that have not taken up the new DNA but won’t harm the transformed cells. The embryos that have been transformed are then regenerated into whole plants (see above). The first American chestnuts to be transformed this way contain an oxalate oxidase gene that originates from wheat (reference below).
Our average, transformation efficiency has increased from the 20 transformation events per gram we reported in 2006 to approximately 170 stable embryogenic transformation events per gram of somatic embryo tissue in 2013. We have transformed 325 individual embryogenic events using 36 different gene constructs. Close to 100 of these events carrying 23 of the different gene constructs have been regenerated into whole plants. As of the fall of 2013, we have planted 1275 transgenic chestnut trees at eight sites in New York State and one in Virginia. Based on field-trial inoculations, greenhouse small-stem inoculations and detached-leaf assays, three transgenes that produce stronger resistance to chestnut blight than non-transgenic American chestnut have been identified. This resistance can be intermediate between American and Chinese chestnut, approximately equal to, or even higher than the resistance naturally found in Chinese chestnut, depending on the transgene and the event.


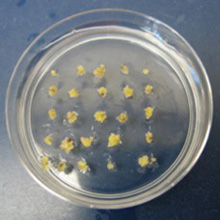

The pictures above follow the process from inoculating medium with the Agrobacterium (fig. 1), co-cultivating the Agrobacterium with chestnut embryos (fig. 2), desiccating the Agrobacterium/embryo mix and then transferring the embryos to Agro Kill medium (fig. 3), and finally the selection of transgenic embryo clumps using a periodic immersion bioreactor (fig. 4). The embryos are then multiplied and regenerated into whole plants as described in the process above.
- American Chestnut tree extended interviews (video)
- Improve Your World with SUNY ESF #36: Chestnut Tree Restoration (video)
Polin L.D., H. Liang, R. Rothrock, M. Nishii, D. Diehl, A. Newhouse, C.J. Nairn, W.A. Powell, and C.A. Maynard. 2006. Agrobacterium-mediated transformation of American chestnut (Castanea dentata (Marsh.) Borkh.) somatic embryos. Plant Cell Tissue and Organ Culture. 84: 69-79
Maynard C.A., L.D. McGuigan, A.D. Oakes, B. Zhang, A.E. Newhouse, L.C. Northern, A.M. Chartrand, L.R. Will, K.M. Baier, and W.A. Powell (In Press). Chestnut, American (Castanea dentata (Marsh.) Borkh.) Will appear in: K. Wang ed. Agrobacterium Protocols. Third Edition
